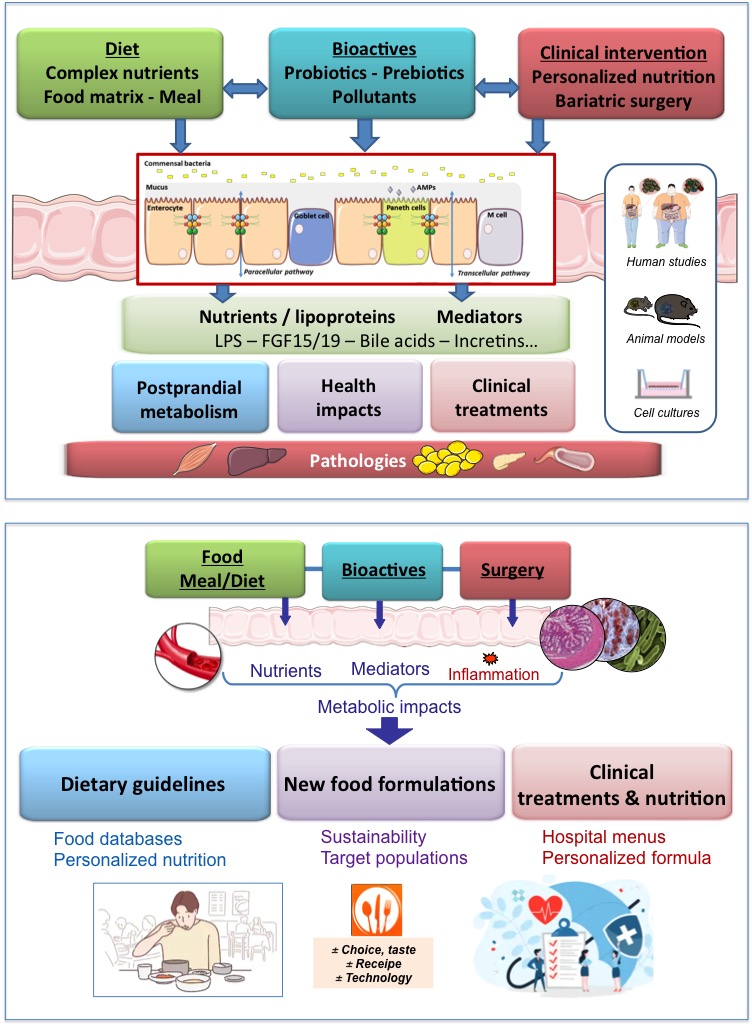
Our research includes:
- Dietary polar lipids of animal (milk polar lipids, sphingolipids) and plant-based (lecithins, galactolipids, etc.) origin.
- “Slow vs. Fast” lipids (more or less emulsified and/or rigid vs. liquid) in food matrices.
- Prebiotics, slowly digestible starch, polyphenols, bioactive lipids and multifunctional synergistic strategies targeting different components of cardiometabolic risk
- Probiotics for the treatment of type 2 diabetes and other metabolic diseases, chronic kidney disease, and for growth stimulation
- Metabolic mediators of intestinal origin and their impacts and modulation through nutrition and metabolic status: FGF19 as prevention agent of sarcopenia, bile acids, metabolic endotoxemia, identification of new biomarkers of food-microbiota interactions.
- Personalized bariatric medicine, surgical innovation and safety, dietary behaviour and nutritional management strategies.
- Impacts on adipose tissue and stem cells: immunological and metabolic phenotyping, understanding of mechanisms and functional regulation.
- Mathematical modelling of lipid absorption and postprandial response.