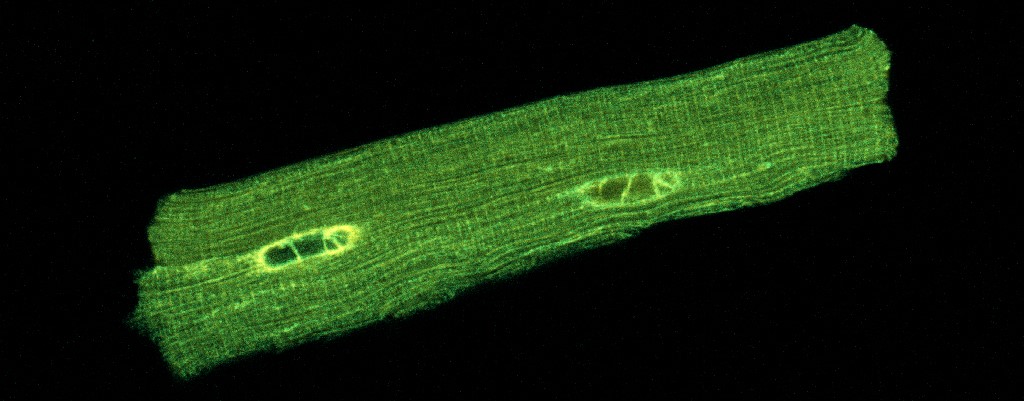
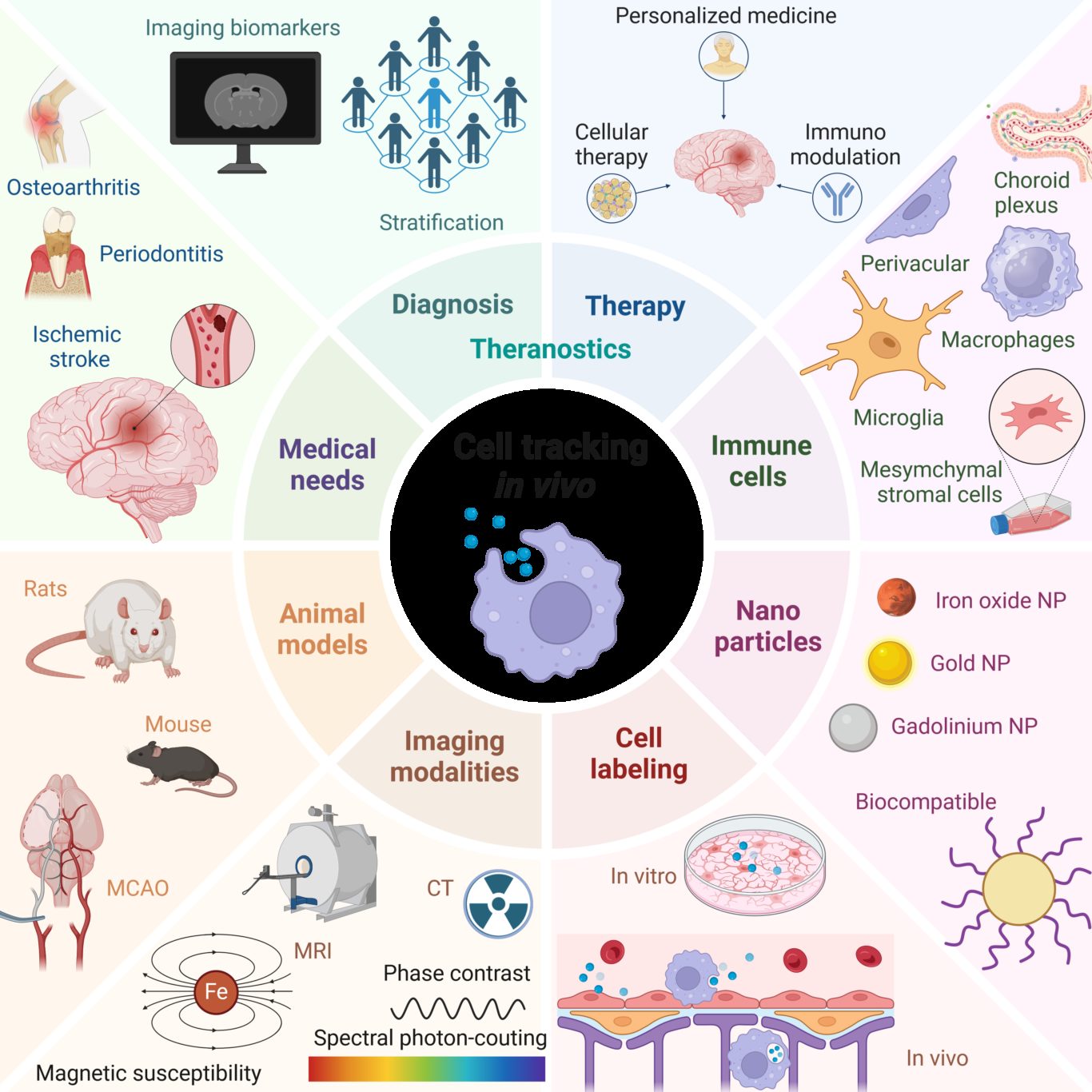
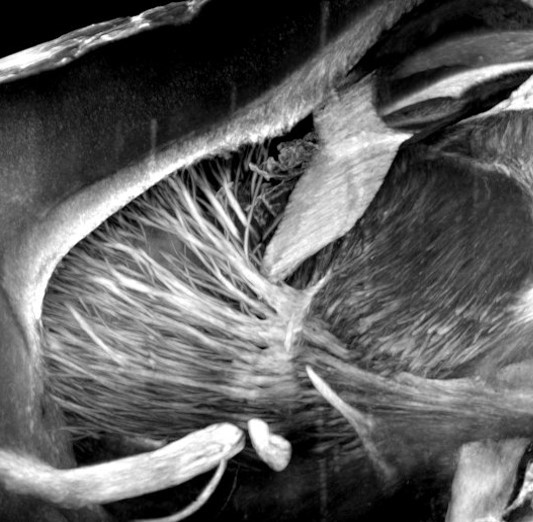
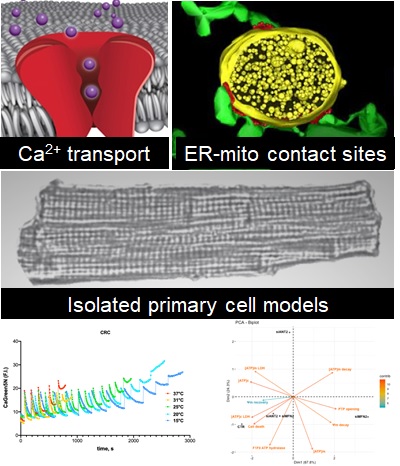
Aim 1. Imaging cell death and inflammation
Marlène Wiart is in charge of the axis.
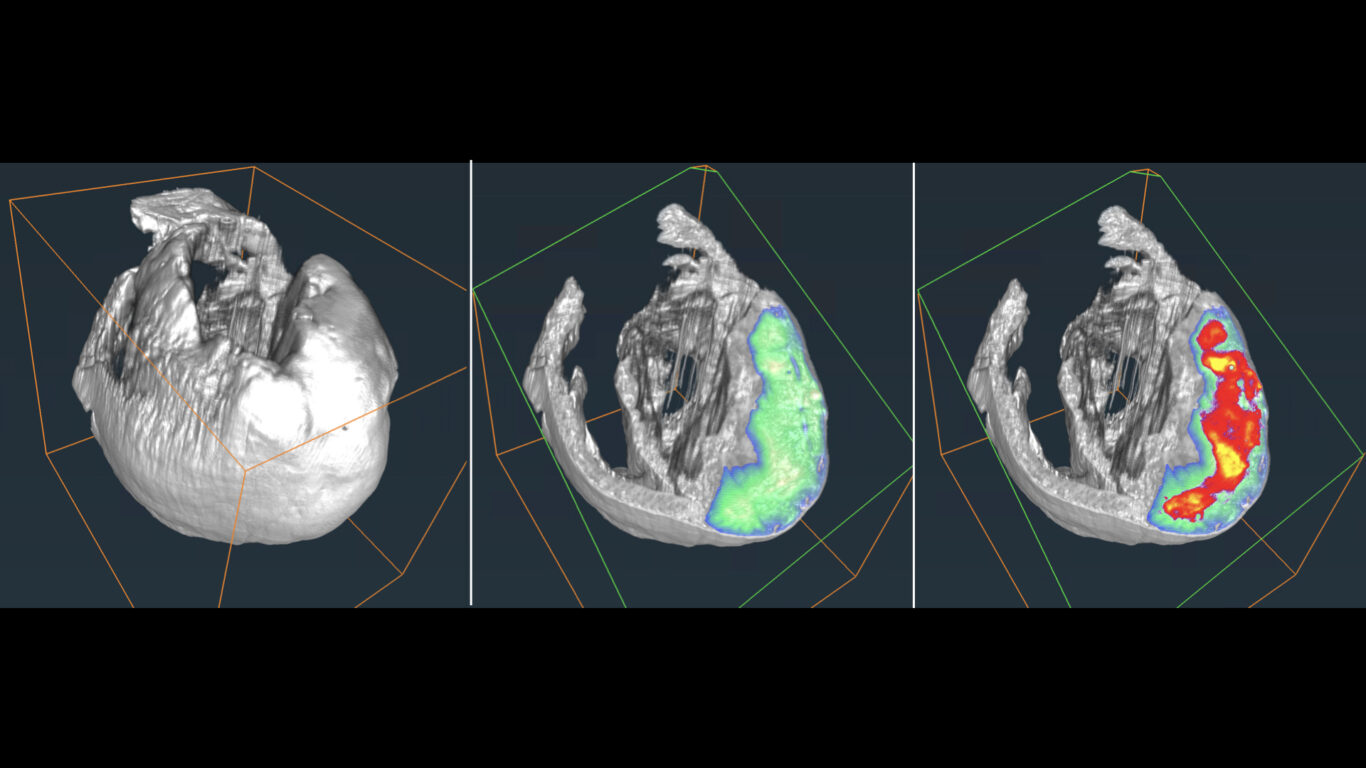
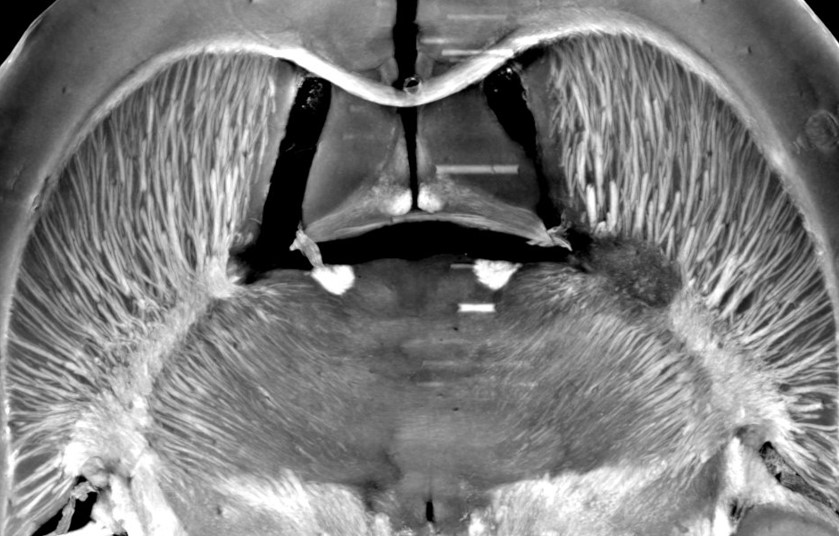
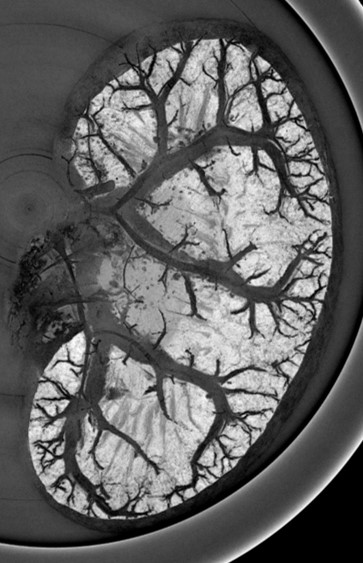
The management of ischemic stroke patients has been revolutionized in the last 5 years with the advent of mechanical thrombectomy, in complement to thrombolysis, to de-obstruct the arteries. This significant therapeutic progress should not hide the fact that ischemic stroke remains a very debilitating pathology: only 45% of thrombectomized patients are independent in their daily life at 3 months. Moreover, 30 to 50% of thrombectomy patients still have poor clinical outcome despite a successful recanalization, the so-called “futile recanalization”. There is therefore an urgent need to propose neuroprotection strategies in order to protect the brain from ischemia and reperfusion damages. To date, none of the therapeutic approaches validated in the pre-clinical arena have been translated into the clinics. One of the reasons for this failure of translational research is a complex physiopathological cascade that is still not completely understood, but also the preclinical methods used are not entirely transposable. We here propose to reveal the determinants of ischemia-reperfusion injuries via molecular imaging tools that characterize and monitor tissue damage in a longitudinal and individual manner. To this aim, we build upon our long-term expertise for multimodal (MRI, PET, CT, US) imaging of edema, perfusion, metabolism and inflammation, in particular macrophage imaging and blood brain barrier dysfunctional. The challenge is to better evaluate and select the treatments to be tested in clinical trials, using a robust and translational methodology. Developing (diagnostic and theranostic) companions imaging tools and their associated imaging biomarkers should favor the clinical transfer of our findings. These imaging tools may further be extended to ischemia/reperfusion syndromes occurring in other organs, such as the heart and the kidney. In conclusion, our ambition is to contribute bridging the gap between pre-clinical and clinical research through in vivo molecular imaging, with the hope, at term, to foster a personalized patient management.