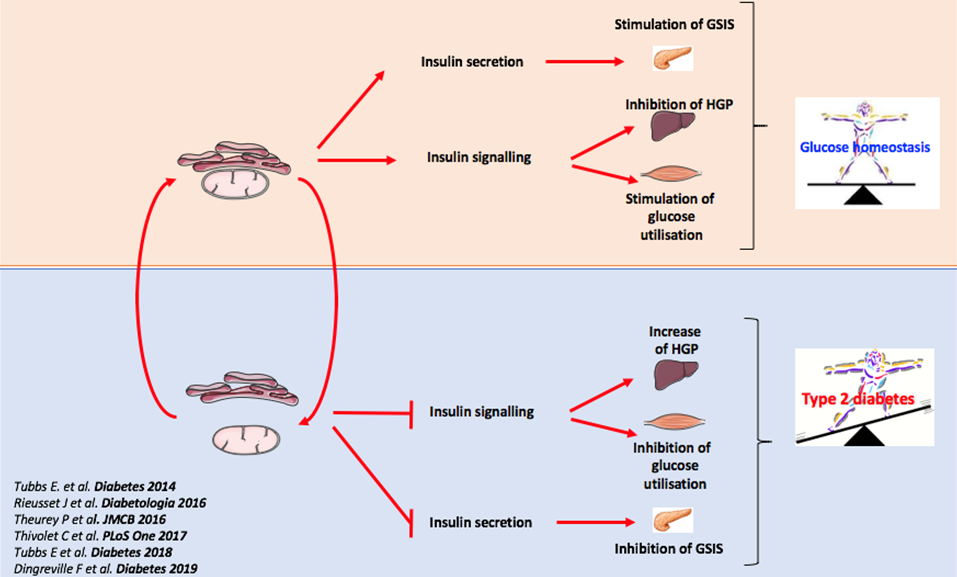
In this context, our pioneering work over the last 5 years has shown that MAMs are new hubs for nutrients and hormones signalling, playing a crucial role in metabolic flexibility and in the regulation of glucose and lipid metabolisms in the liver, skeletal muscle and pancreatic ß cells (Rieusset J, Cell Death Dis 2018; Theurey P & Rieusset J, Trends Endocrinol Metab 2017). In addition, we have identified ER-mitochondrial miscommunication as a novel mechanism of hepatic and muscle insulin resistance (Tubbs E et al. Diabetes 2014; Tubbs E et al Diabetes 2018) and of pancreatic β cell dysfunction (Dingreville F et al. Diabetes 2019), pointing MAMs as a key player of glucose homeostasis. These data have been partially confirmed in humans (Tubbs E et al. Diabetes 2018, Thivolet C PLoS 2017). Therefore, targeting MAMs could constitute an interesting strategy to stimulate insulin action and secretion and to improve glucose homeostasis during T2DM (Figure 2). MAMs could also control lipid metabolism (Bassot A et al. Biochim Biophys Acta Mol Cell Biol Lipids 2021) and miscommunication between these two organelles could also be involved in the pathophysiology of NAFLDs and dyslipidemias.
MERISM TEAM : « Mitochondria-ER Interactions and Signalling in Metabolic health and diseases »
Objectifs de l'équipe
Contact sites between the ER and mitochondria, called MAMs (mitochondria-associated membranes), have recently emerged as key regulators of mitochondrial bioenergetics and of several signalling pathways, allowing cells to adapt their metabolism to environmental changes and maintain cell homeostasis.
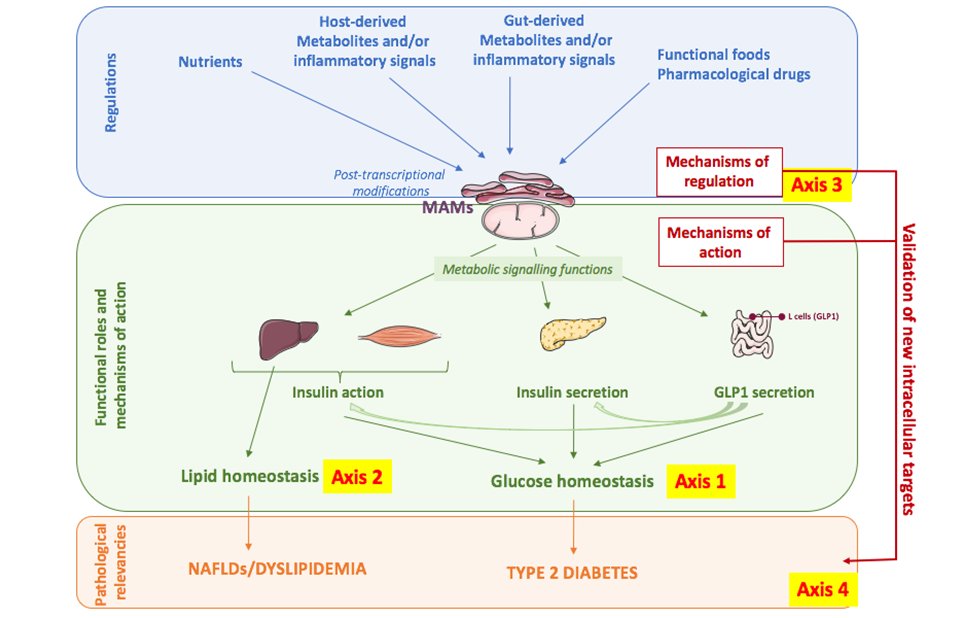
The general objective of our research program will be to better characterize the functional roles of MAMs in the control of glucose and lipid homeostasis and to identify new actors or regulators of MAMs that could represent new targets to improve metabolic diseases, in particular T2DM, NAFLDs and / or dyslipidemias (Figure 3).
More specifically, our specific objectives will be:
1) To characterize the physiological significance of MAMs in the control of glucose and lipid metabolisms and to study the underlying molecular mechanisms.
In addition to the role of MAMs in the control of insulin action and secretion, we plan to investigate a new role of MAMs in nutrient-induced GLP1 secretion in gut L cells, as well as in the action of GLP1 in the liver and pancreas.
Regarding lipid metabolism, we want to determine the impact of MAMs in the intracellular storage of lipids via a control of de novo hepatic lipogenesis, the oxidation of mitochondrial fatty acids, and/or the secretion of lipoproteins. In addition, we want to determine whether organelle miscommunication participates to hepatic steatosis-associated with NAFLDs and to the progression to NASH, but also in the defects of lipolysis of lipoproteins rich in TG during dyslipidemia.
Finally, we plan to develop and use nuclear magnetic spectrometry (NMR) to further characterize the metabolic functions of MAMs in liver and β cells, and to analyze their alterations in metabolic diseases.
2) To identify new nutritional or pharmacological regulators of AMS and their functional impacts.
Since MAMs are regulated by some nutrients, we propose that specific nutritional approaches could improve glucose homeostasis by targeting MAMs. We therefore want to better understand the physiological regulations of MAMs and their impact on the metabolic flexibility of the liver and on metabolic homeostasis. More specifically, we will study the effects of certain amino acids, pro-inflammatory cytokines and bioactive metabolites produced by the intestinal microbiota and altered during metabolic diseases. We also want to better understand by which post-transcriptional modifications are dynamically regulated MAMs.
3) To validate MAMs as a target for the treatment of metabolic diseases.
We want to validate all of our concepts in humans by investigating MAMs in both tissues and peripheral blood mononuclear cells (PBMCs) of different patients. In parallel, we will carry out screenings of different functional foods (in collaboration with manufacturers) and/or pharmacological drugs, which will then be validated in preclinical models of obesity, T2DM and NAFLDs, and ultimately in humans.
Skills
- Preclinical murine models of T2DM and NAFLD
- Primary murine and human cells in culture (hepatocytes, myotubes and islets)
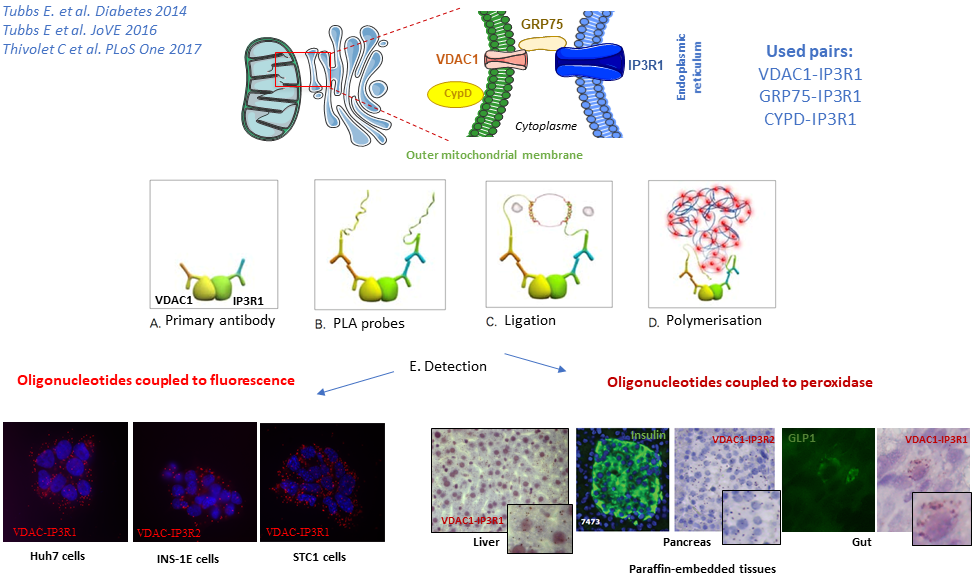
- Structural and functional analysis of mitochondria-RE interactions. In particular, we have developed and validated a new method to detect and quantify ER-mitochondria interactions by in situ Proximity ligation assay (PLA), via the visualization of VDAC1-IP3R1, Grp75-IP3R1 or CypD-IP3R1 interactions in fixed cells. or paraffin coated tissues (Figure + JoVE video).
- We modulate MAMs via adenoviral approaches allowing the overexpression of spacer (FATE1, provided by E Lalli, France) and linker (provided by G. Hadjnokzky, USA).
- Analysis of the action and secretion of insulin
- Proteomic analysis
- Lipidomic analysis
- Strong complementarity between fundamental research and clinical investigations favoring translational research
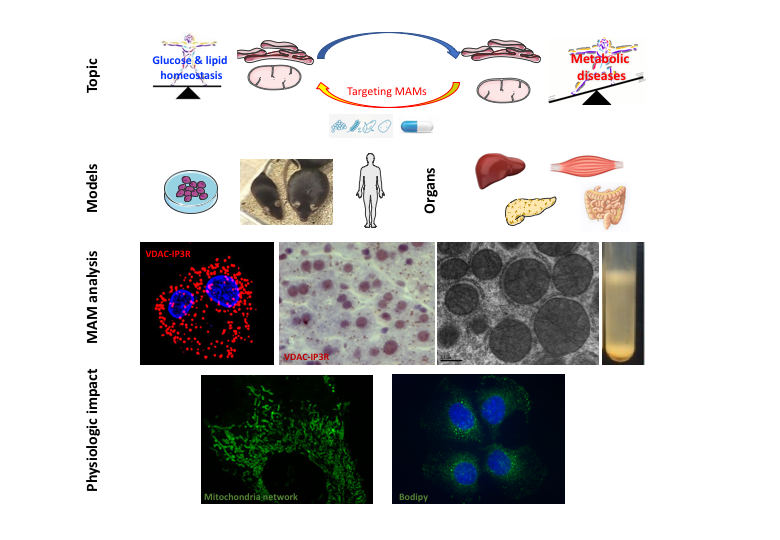
Biological resources
- Tissues from obese and diabetic mice
- Primary murine and human cells in culture (hepatocytes, myotubes and islets)
- Fractions of mitochondria, ER and MAMs from liver and skeletal muscle from obese and diabetic mice
- Sections of human liver and pancreas (nPOD network)
- Human PBMCs

